CTC-AE+
 다운로드수 39
다운로드수 39-
0 (0명)
| 분류 | 의료 |
|---|---|
| 버전 | 버전 3.1 |
| 업데이트 | 2021년 5월 17일 |
| 용량 | 14.9MB |
| 앱결제 | ₩4,400 |
| OS | iPhone: iOS 11.2 이상 필요 / iPad: iPadOS 11.2 이상 필요 / |
| 디자인 | iPad용으로 디자인됨 |
| 금주 다운수 | 0 |
| 누적 다운수 | 39 |
| 다운로드 | |
|
!소프트웨어 사용범위 위반 시
법적 책임을 질 수 있습니다. |
|
상세정보
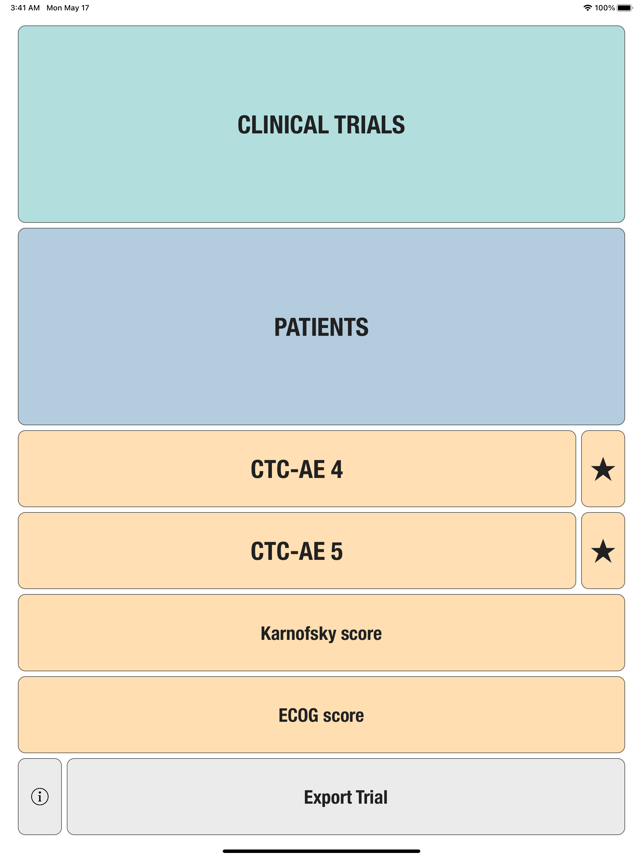
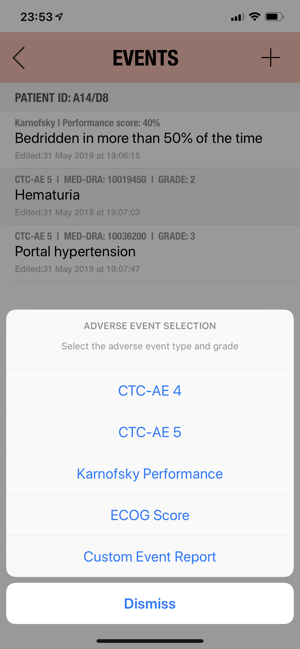
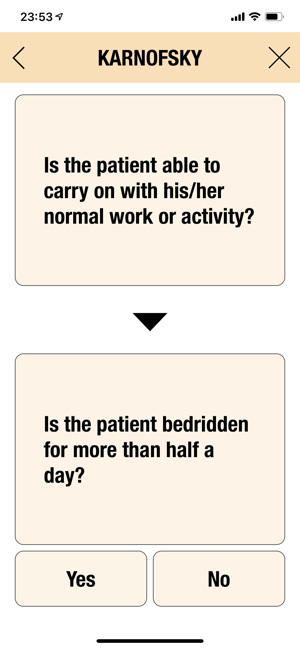
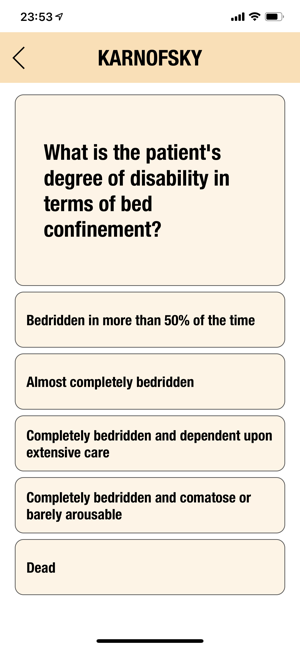
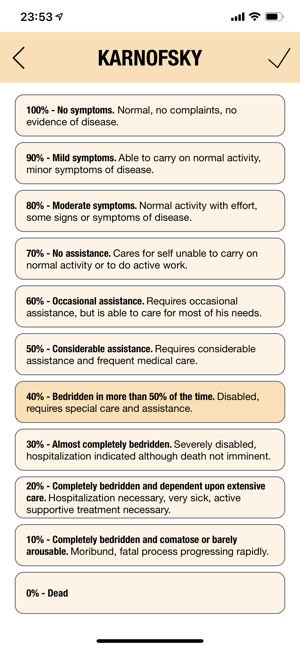
CTC-AE+ is a browsable reference to the CTC-AE list of adverse event (AE) terms commonly encountered in oncology, plus a portable Adverse Event Logger to keep track of all adverse events during a clinical study.The CTC-AE 4 and CTC-AE 5 have been developed from the earlier vocabulary known as CTC (Common Toxicity Criteria). Each AE term is defined and accompanied by a grading scale that indicates the severity of the adverse event. All AE terms are organized by the System Organ Classes (SOCs) defined by the Medical Dictionary for Regulatory Activities (MedDRA).Adverse events are common phenomena affecting patients being treated for cancer. With the availability of new agents and the multimodality interventions, it is critical to monitor systematically the AEs that are linked to oncology research.CTCAE is fundamentally intended to be an agreed upon terminology for the designation, reporting, and grading of AEs that occur in oncology research.########################CTC-AE serves severa
새로운 기능
Added CTC-4 and CTC-5 bookmarks for the most common adverse events.
저작권
© Arpacore B.V.
리뷰
- 소프트쉐어 신규 소프트웨어 추가 안내 2025.01.17
- 소프트쉐어 서비스 이용 가이드 업데이트 안내 2025.01.17
- 소프트쉐어 웹사이트 리뉴얼 안내 2025.01.17